|
INTRODUCTION
GENETICALLY MODIFIED ORGANISMS
LEGISLATON
BY SUBJECT
BY USAGE
COMPETENT AUTHORITIES
PROFESSIONAL ASSISTANCE
GMOS IN SLOVENIA
EVENTS
LEGISLATION
GMOS IN EU
CARTAGENA PROTOCOL
BCH CENTRAL PORTAL
NATIONAL BCH
USEFUL LINKS
|
|
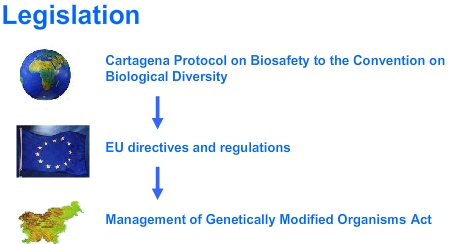
LegislationThe principal aim of the biosafety system is effective supervision of the handling of GMOs. The biosafety system establishes the necessary infrastructure and decision-making procedures, which enable the transparency of decision-making regarding the handling of GMOs with the integration of both professional as well as the greatest possible scope of interested public. The legislative and administrative framework of biosafety for the area of GMOs in Slovenia is established in accordance with the legal order of the EU and the international Cartagena Protocol on Biosafety.
The Management of Genetically Modified Organisms Act (Official Gazette of the RS 67/02, unofficial translation) adopted in July 2002, the implementation of which is within the competency of the Ministry of the Environment and Spatial Planning, is a horizontal act, as its implementation enables a safe use of GMOs or products containing GMOs or which are composed of them or their combinations with the requirement for preliminarily formed evaluation of risk for the environment and people?s health. In this part, it is connected with the regulations from the area of health and agriculture. The Act regulates the handling of GMOs and determines measures for prevention and decrease of possible harmful effects on the environment, especially with regards to the preservation of biodiversity, and people?s health, which could occur while working with GMOs in closed systems, by intentional release into the environment or by placing GMOs or products containing GMOs or which are composed of GMOs or their combinations, on the market. The Act reflects provisions of the EU regulations (of the Directive 90/219/EEC, Directive 98/81/EC and Directive 2002/18/EC) and some provisions of the Cartagena Protocol on Biosafety to the Convention on Biological Diversity, which Slovenia ratified on October 22, 2002 (Official Gazette of the RS 89/02).
After entering the EU, it was necessary to include into the act provisions relating to the inclusion of competent authorities of the EU and competent authorities of the EU member states into the procedures of intentional release of GMOs and of placing products on the market, and bring it into line with new regulations of the EU Parliament and Council relating to the handling of GMOs, which were accepted after the adoption of the Management of Genetically Modified Organisms Act. In July 2004, the National Assembly accepted the Act Amending the Management of Genetically Modified Organisms Act (Official Gazette of the RS 73/2004, unofficial translation).
In 2005, the official consolidated text of the Management of Genetically Modified Organisms Act (ZRGSO-UPB1) (Official Gazette of the RS 23/2005) was published. In 2010 Management of Genetically Modified Organisms Act was amended (Official Gazette of the RS 21/10). Amendment includes provisions of the new Directive 2009/41/EC on the contained use of genetically modified micro-organisms and brings new structure of the Slovene Scientific Committees for GMOs.
Provisions of the Management of Genetically Modified Organisms Act referring to putting products on the market, to export and transit of GMOs and products are not valid for:
|